|
1) Golf, S. 2011. Pharmakologie und Bioverfügbarkeit von Magnesiumverbindungen. Pharmazeutische Zeitung.
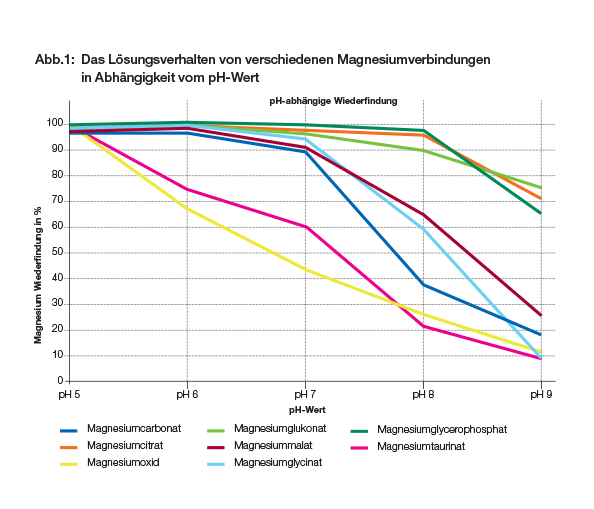
2) Kasel, U., Rempfer. N. 2013. PH-abhängiges Lösungsverhalten. Magnesiumverbindungen im Vergleich. Biogena inside Mineralienschau.
3) Niestroj, I. 2000. Praxis der Orthomolekularen Medizin. Physiologische Grundlagen, Therapie mit Mikro-Nährstoffen.
4) Takase, B. et al. 2004. Effect of chronic stress and sleep deprivation on both flowmediated dilation in the brachial artery and the intracellular magnesium level in humans. Clin Cardiol 27(4):223-7.
5) Maier, J. A. et al. 2004. Low magnesium promotes endothelial cell dysfunction: implication for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta. 1689(1):13-21.
6) Rodriguez-Moran, M., Guerrero-Romero, F. 2007 Serum magnesium and C-reactive protein levels. Arch Dis Child.
7) Almoznino-Sarafian, D. et al: Magnesium and C-reactive protein in heart failure: an anti-inflammatory effect of magnesium administration? Eur J Nutr. 46(4):230-7.
8) Lechner, W. et al. 2001. Reduktion des diastolischen Blutdruckanstiegs in der Schwangerschaft durch Magnesium. J Hyperton. (1):30-4.
9) Fuentes, J. C. et al. 2006. Acute and chronic oral magnesium supplementation: effects on endothelial function, exercise capacity, and quality of life in patients with symptomatic heart failure. Congest Heart Fail. 12(1):9-13.
10) Pokan, R. et al. 2006. Oral magnesium therapy, exercise heart rate, exercise tolerance, and myocardial function in coronary artery disease patients. Br J Sports Med. 40(9):773-8.
11) Assarzadegan, F. et al. 2016. Serum concentration of magnesium as an independent risk factor in migraine attacks. International Clinical Psychopharmacology. 31(5):287-292.
12) Köseoglu, E. et al. 2008. The effects of magnesium prophylaxis in migraine without aura. Magnes Res. 21(2):101-8.
13) Facchinetti, F. et al. 1991. Oral magnesium successfully relieves premenstrual mood changes. Obstet Gynecol. 78(2):177-81.
14) Kia, A. S. et al. 2015. The association between the risk of premenstrual syndrome and vitamin D, calcium, and magnesium status among university students: a case control study. health promotion perspectives health promot perspect. 5(3):225-230.
15) Siegler, J. C. et al. 2008. Pre-exercise alkalosis and acid-base recovery. Int J Sports Med. 29(7):545-51.
16) Cinar, V. et al. 2007. Effects of magnesium supplementation on blood parameters of athletes at rest and after exercise. Biol Trace Elem Res. 115(3):205–12.
17) Worlitschek, M. Praxis des Säure-Basen-Haushalts. Grundlagen und Therapie. 2008.
18) Oöpik, V. et al. 2003. Effects of sodium citrate ingestion before exercise on endurance performance in well trained college runners. Br J Sports Med. 37(6):485–489.
19) König, D. et al. 2009. Effect of a supplement rich in alkaline minerals on acidbase balance in humans. Nutr J. 8:23.
20) Laires, M. J., Monteiro, C. 2008. Exercise, magnesium and immune function. Magnes Res. 21(2):92-6.
21) helm, S. M. et al. 2013. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 6(4):443-51.
22) S. et al. 2013. Clinical redictors associated with proton pump inhibitor-induced hypomagnesemia. Am J Ther.
23) El-Charabaty E. et al. 2013. Effects of proton pump inhibitors and electrolyte disturbances on arrhythmias. nt J Gen Med. 6:515-8.
References Interactions
Stargrove, M. B. et al. Herb, Nutrient and Drug Interactions: Clinical Implications and Therapeutic Strategies, 1. Auflage. St. Louis, Missouri: Elsevier Health Sciences, 2008.
Gröber, U. Mikronährstoffe: Metabolic Tuning –Prävention –Therapie, 3. Auflage. Stuttgart: WVG Wissenschaftliche Verlagsgesellschaft Stuttgart, 2011.
Gröber, U. Arzneimittel und Mikronährstoffe: Medikationsorientierte Supplementierung, 3. aktualisierte und erweiterte Auflage. Stuttgart: WVG Wissenschaftliche Verlagsgesellschaft Stuttgart, 2014.
|